Eli Lilly and Company (NYSE: LLY) recently announced promising results from its SUMMIT phase 3 trials, focusing on the efficacy of the tirzepatide injection.
The positive findings have sparked investor interest, driving the stock up by 3% today.
These results are not only significant for Eli Lilly but also hold potential for millions of patients suffering from heart failure with preserved ejection fraction (HFpEF) and obesity.
Promising trial results
The SUMMIT phase 3 trial results revealed that tirzepatide reduced the risk of heart failure outcomes by 38% compared to a placebo. Additionally, all key secondary endpoints were successfully met, including:
It reduced the risk of heart failure outcomes by 38% compared to placebo.
All l key secondary endpoints were met successfully. These included exercise capacity, mean body weight reduction from baseline at 52 weeks, and reduction in the inflammation marker high-sensitivity C-reactive protein.
For the efficacy estimand, it led to a 15.7% weight reduction compared to 2.2% for placebo.
For the treatment regimen estimand, it led to a 13.9% reduction in body weight compared to 2.2% for placebo.
Why is this result significant?
The result carries weight because HFpEF (heart failure with preserved ejection fraction) accounts for 50% of all heart failure cases. In the US, 60% of those also suffer from obesity.
It is a condition where the left pumping chamber of the heart becomes stiff and is unable to fill normally. Thanks to tirzepatide, this problem can finally be addressed.
The company will now submit the SUMMIT study findings to the FDA and hope for a positive response from them. If the drug is approved, tirzepatide could become the cornerstone of Eli Lilly’s portfolio and substantially boost revenue.
CEO says shortage of tirzepatide should end soon
CEO David Ricks had more good news for the investors today as he claimed the shortage of tirzepatide should end soon. He made this revelation during an interview with Bloomberg.
Some doses of LLY’s weight loss drugs Zepbound and Mounjaro have been on FDA’s shortage list for some time now. In fact, Mounjaro is on that list since since 2022.
The company had initially stated in April that it expects the supply to remain tight. However, the CEO’s comments have increased investor confidence and hopes.
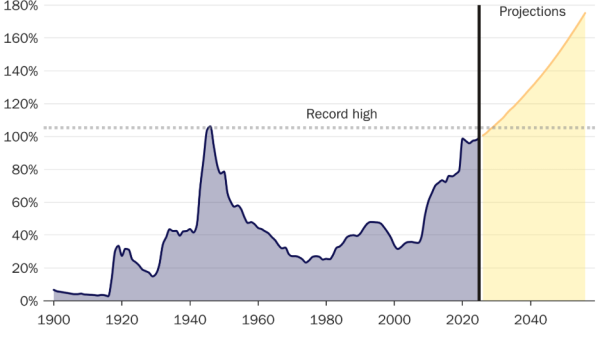
What does the chart say?
LLY stock has been on a downslide recently as the US market corrects across the board. The announcement of the drug trial comes at a time when the stock was about to test a key support level as shown in the chart below.
The trendline that started over a year ago was about to be approached but the stock price never reached there. Traders will probably still wait for a retesting of that line but investors, on the back of above positive developments will hope that the retesting isn’t needed and the stock can continue its upward journey.
As the market tumbles today, investors will find comfort in the fact that LLY stock is still up 3%. Only time will tell if that’s temporary though.
The post Eli Lilly’s tirzepatide injection results could trigger another bull run: Here’s why appeared first on Invezz